|
|
[Previous Lecture] [Next Lecture] [Course Outline] [General Info] [Study Guide]
When a metal is not in equilibrium with a solution of its ions, the electrode potential
differs from the equilibrium potential by an amount known as the polarisation. Other terms having equivalent meaning
are overvoltage and overpotential. The symbol commonly used for polarisation is ![]() .
.
Polarisation is an extremely important corrosion parameter for which relationships have been established which enable useful statements to be made about the rates of corrosion processes.
In practical situations, polarisation is somtimes defined
as the potential change away from some other arbitrary potential (often the mixed potential or the free corrosion
potential, see section 4.9). Polarisation can also be expressed by ![]() E.
E.
![]() E=E -
Ecorr=
E=E -
Ecorr=![]()
Consider the process described in eqn. (4.38). In the last section we saw that corrosion rate and current density are directly related. At the beginning of this chapter we said that corrosion rate, v, could be expressed:
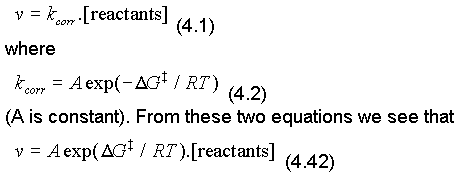
At equilibrium, the rate of the forward (anodic) reaction is ia, and equals the rate of the reverse (cathodic) reaction, ic. (Remember that io = ia = ic at equilibrium.) It is usually possible to treat the concentration of reactants (e.g. the solid metal, for the forward reaction) as constant, and we shall incorporate the term into a new constant, Ao. Thus, if we consider the rate of the forward reaction for which the activation free energy is shown , we can write eqn. (4.42) as:
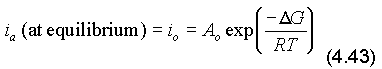
When the forward reaction is faster than the reverse reaction (ia > ic) and an overall corrosion process occurs, equilibrium is destroyed and the free energies of the metal and its ions are at different levels, Fig. 4.6(a).
The deviation from the equilibrium potential, the polarisation, is the combination of an anodic polarisation on
the metal and a cathodic polarisation of the environment. (Compare Fig. 4.6(a) and (b): the energy of the metal has increased
and that of the environment has decreased.) These potential deviations away from the equilibrium value may or may
not be equal, and we shall assume for the moment that they are not. The changes are redrawn in Fig.
4.8.
If we define the total polarisation, then we can define the anodic polarisation and the cathodic polarisation. Note that in Fig. 4.8, the polarisations have been converted into free energies by multiplication by the factor, zF, as in the Faraday equation, (4.13). This enables us to determine the new activation energy for the anodic reaction, because the energy state of the metal has increased and the activation energy reduced. Thus we can write:
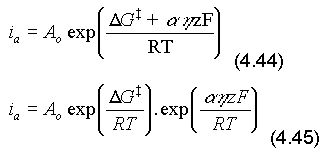
(Note that, similarly, the activation energy of cations, Mz+, being converted into M, i.e. the cathodic reaction, has increased. Substituting eqn. (4.43) into eqn. (4.45)
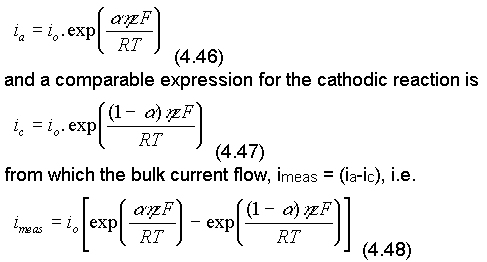
This equation is known as the Butler-Volmer equation and is much used in this subject.
Now let
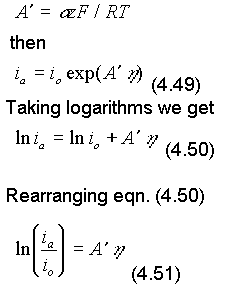
Converting to base 10 logarithms and rearranging eqn. (4.51)
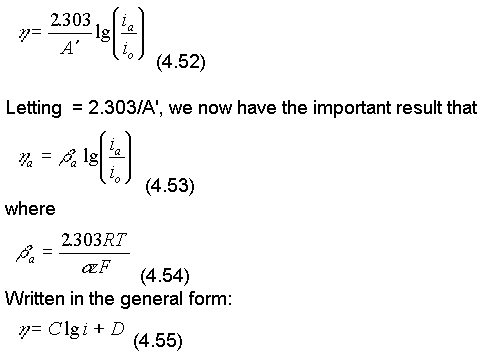
the equation is known as the Tafel equation, whilst more specifically for the anode process:
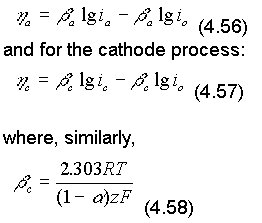
The constants a and c, are called the anodic and cathodic beta or Tafel constants.
Examination of the Tafel equation in its form of eqn. (4.55) tells us immediately that a graph for either of the
two processes gives a straight line with a slope equal to the respective constant. The intercept, D, is shown.
In the context of the Nernst equation (4.17) we found that 2.303RT/F has the value 0.059 and, suitably modified,
sensible values for Tafel constants can readily be evaluated. As we have seen, z takes values 1, 2 or 3 and alpha
is usually about 0.5. In practice, a value of +/- 0.03 to +/- 0.1 volts (or +/- 30 to +/- 100 mV) per decade of
current density is common.
The three-electrode cell is the standard laboratory apparatus for the quantitative investigation of the corrosion properties of materials. It is a refined version of the basic wet corrosion cell and a typical example is illustrated in Fig. 4.14. It can be used in many different types of corrosion experiments. First we shall examine the components in more detail.
The working electrode is the name given to the electrode being investigated. It is useful, though not essential,
if the electrode is designed to have a surface area of at least 100 square millimetres (1 cm2); current measurements
can then be more readily converted into current densities, which should be used in calculations. Laboratory and
field experiments are, however, often better performed using larger electrodes, if convenient. We use the term
'working electrode' rather than 'anode' because we are not limited to investigations of anodic behaviour alone:
cathodic behaviour can also be examined.
Practical working electrodes can be constructed in a variety of ways. A simple method is to mount a small specimen in cold-setting resin, Fig. 4.15(a). Electrical connection must be made to the specimen, and this can be done with solder or spot weld on the reverse side before mounting. After mounting, specimens are often ground and polished, as for metallographic examination. If this technique is used, the surface will be 'activated', in other words, passive films may have been either removed entirely or just changed from the 'as-received' condition. This should always be borne in mind. Obviously, if the original passive film is part of the corrosion investigation, no pre-treatment should be used. In fact, this is the biggest single reason for discrepancies between 'lab' and 'field' data. Surfaces in real engineering systems are most often 'as-received' from manufacture and are not similar to specimens prepared for metallography.
Although these specimen configurations are adequate for most purposes, accurate work may require a more carefully designed method of mounting. Two designs which are commercially available are illustrated in Fig. 4.15(b) and Fig. 4.15(c). Specimens in the form of thin discs of approximately 15 mm diameter, or cylinders of about 10 mm diameter x 10 mm length, can be quickly assembled into working electrodes with well-defined characteristics.
The counter (auxiliary) electrode is the name given to the second electrode which is present specifically to carry the current in the circuit created by the investigation: it is not required for measurements of potential. Usually, a carbon rod is used, but it can be any material which will not introduce contaminating ions into the electrolyte. Platinum or gold can also be used with success, especially if space is at a premium, when smaller electrodes can be used; titanium is also suitable.
The reference electrode is present to provide a very stable datum point against which the potential of the working electrode can be measured. It cannot itself carry any more than the most negligible current. If it did, it would participate in the cell reactions and its potential would no longer be constant, hence the requirement for the counter electrode. By far the most convenient reference electrode to use in such an experiment is a saturated calomel electrode, SCE.
The external circuit can be varied considerably. The essential components are the following:
A current measuring device.
A potential measuring device.
A source of potential.
Polarisation is simply a shift or change in potential. In reality, many factors may cause
polarisation of a system for better or for worse. Polarisation will affect the rate of corrosion reaction. Tafel
Equation, undoutedly, one of the most important equations in corrosion science and engineering, relates polarisation
(![]() E or
E or ![]() ) to the rate (ia or ic or icorr) of reactions. An experimental
Tafel polarisation curve can be measured using a three-electrode corrosion cell interfaced with a potentiostat.
) to the rate (ia or ic or icorr) of reactions. An experimental
Tafel polarisation curve can be measured using a three-electrode corrosion cell interfaced with a potentiostat.
To reinforce learnings in this lecture read pages 95-100, 111-120 (textbook)
To prepare yourself for the next lecture
read pages 93-95, 100-106 (textbook)
"Principals and Prevention of Corrosion", Denny Jones, 2ed., Printice-Hall,
1996, pp84-86