|
|
[Previous Lecture] [Next Lecture] [Course Outline] [General Info] [Study Guide]
The rate of a reaction is determined by the slowest step - rate determining step.
transport of reactants to the reaction site is relatively fast, activaton process (electron transfer) is the rate determining step.
transport of reactants is the rate determining step.
Take cathodic reduction of H+ as an example:
2H+ + 2e- = H2
At high reaction rates, cathodic reduction reactions deplete the adjacent solution of the dissolved species, H+ ions in this case. The concentration profile of H+ ions is shown schematically in the figure below.
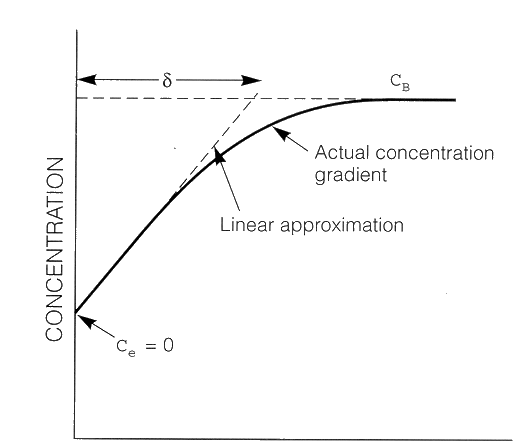
Concentration profile of H+ in solution near the surface of an electrode (cathode).
The process is controlled by diffusion or contrentration polarisation. CB is the H+ concentration
of the uniform bulk solution; Ce is the the H+ concentration at the electrode surface which
is zero for a diffusion controlled process. ![]() is the thickness of the concentration gradient in solution (is also refered to as the Double
Layer Thickness).
is the thickness of the concentration gradient in solution (is also refered to as the Double
Layer Thickness).
Diffusion Limiting Current Density, iL, is a measure of the maximum reaction rate that can not be exceeded because of a limited diffusion rate of H+ in solution:
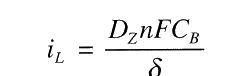
where DZ is the diffusivity of the reacting species, Z (H+) in this example.
iL will be increased if
The relationship between iL and concentration polarisation is:
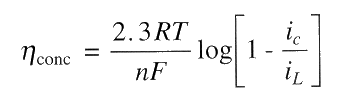
For corrosion, concentration polarisation is primarily for cathodic processes, particularly, for the reduction of dissolved O2 in natural waters. Concentration polarisation for anodic oxidation during corrosion can usually be ignored because an unlimited supply of metal atoms is available at the metal/solution interface. However, when the rate of anodic reaction has become too high (during intentional anodic dissolution by impressed current such as electrochemical machining or electrorefining), concentration polarisation of the anodic reaction is possible as the rate-determining step is the the transport of oxidation products away from the reaction site!
Total cathodic polarisation is the sum of the activation and concentration polarisation:
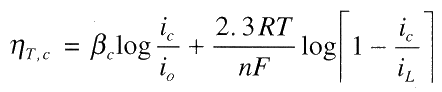
For anodic process, concentration polarisation is usually absent, thus:
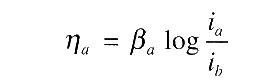
For detailed disscusion of concentration polarisation refer to the following book:
"Principles and Prevention of Corrosion", Denny Jones, 2ed., Printice-Hall, 1996, pp84-86
Double Layer: the non-homogeneous distribution of ions at the metal/solution interface.
The double layer consists of two parts:
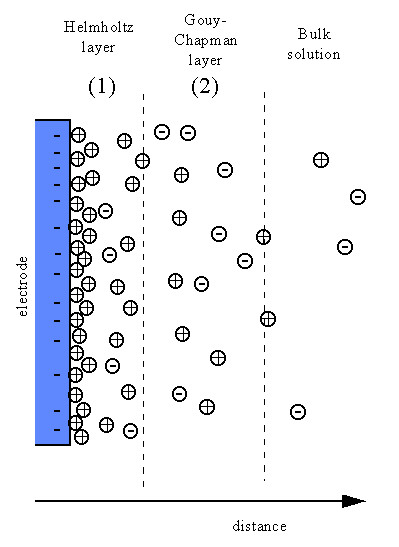
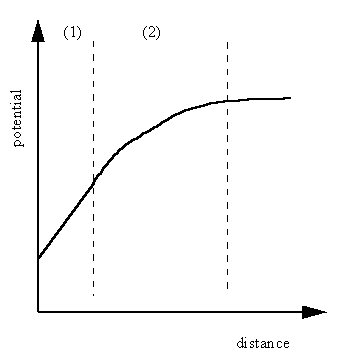
Fig4.7 The distribution of ions at the metal/solution interface and the variation of potential
The following animation shows the process of ionization of metal immersed in water and the formation of the double layer.
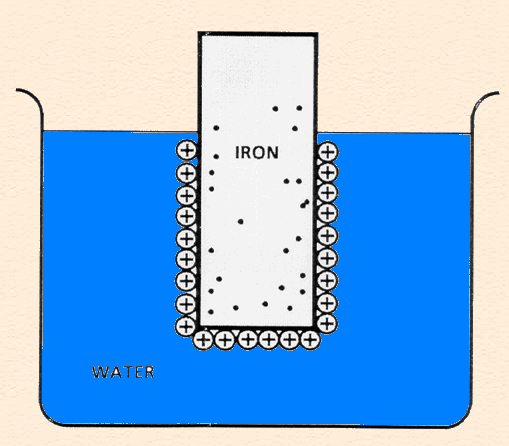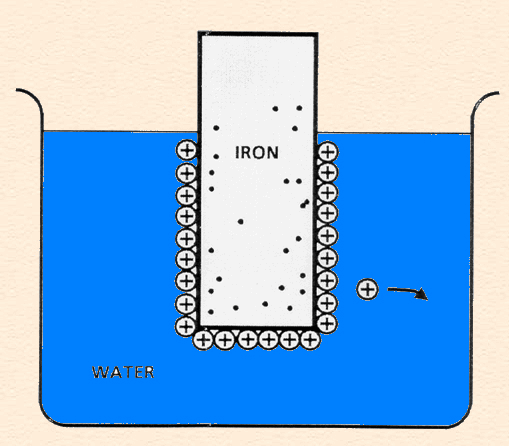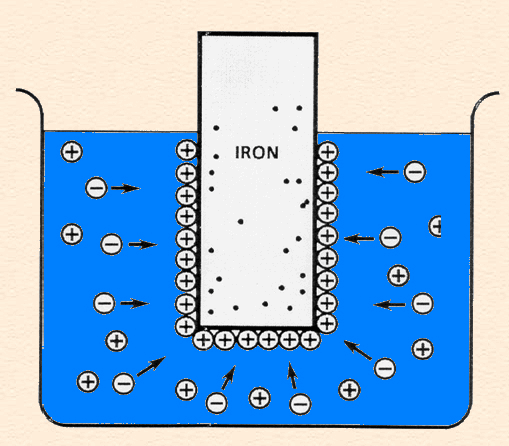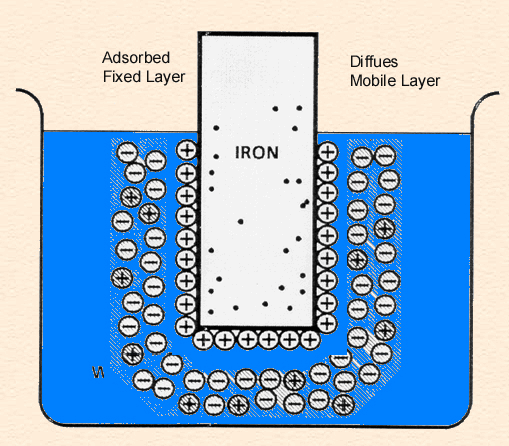
Single electrode (zinc in acid) reactions:
Zn = Zn2+ + 2e- anodic reaction
2H+ + 2e- = H2 cathodic reaction
Each has its own half-cell electrode potential and exchange current density.
The two half-cell electrode potentials cannot coexist separately on the same electrically conductive surface (metal).
Each must polarize or change potential to a common intermediate value, the mixture of the half-cell electrode potentials, most often called the mixed potential.
Other terms such as:
corrosion potential, Ecorr
rest potential
open circuit potential
free corroding potential
all refer to the mixed potential of a corroding system.
Two electrode system:
examples: Zn-Fe
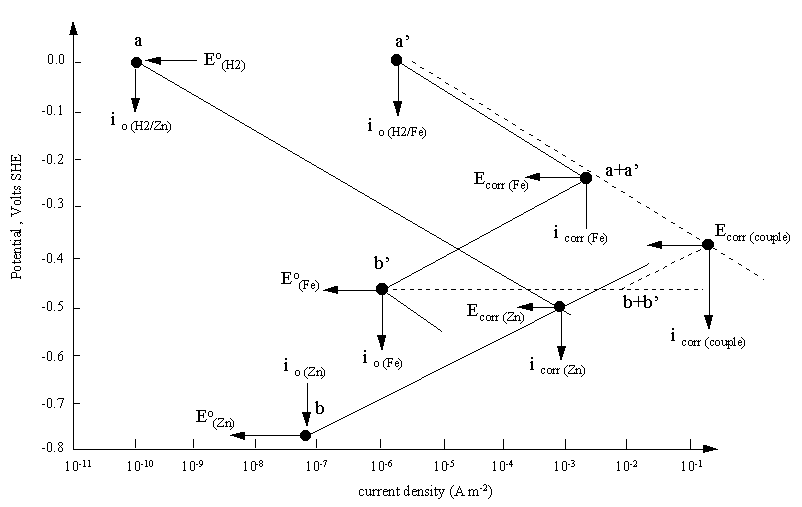
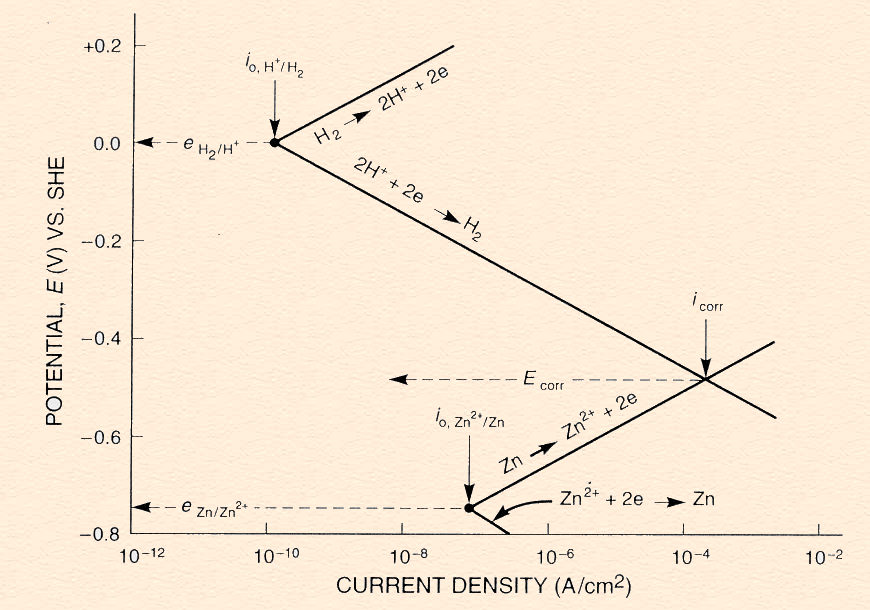
A mixed potential plot for the bimetallic couple of iron and zinc. The diagram
also explains the higher corrosion rate of iron than zinc in hydrochloric acid solution. Despite the more positive
reduction potential of iron, the evolution of hydrogen on iron has a high exchange current density.
The principle of charge conservation applies to the corrosion reaction.
In a corrosion reaction,
The total rate of oxidation must equal the total rate of reduction.
The sum of anodic oxidation currrents must equal the sum of cathodic reduction currrents.
Evans diagram (Mixed Potential plot):
schematic representation of the relationship between thermodynamics (potential) and
kinetics (current) in a corrosion process.
In this lecture, we discussed concentration polarisation, the formation of double layer at the metal/electrolyte interface and the mixed potential theory. Concentration polarisation is most often observed for the cathodic processes in a corroding system. When the availability of cathodic reactants at the cathode/eletrolyte interface is limited, the rate of reaction will be determined by the rate of diffusion (iL). The double layer is an equilibrium arrangement of ions at the metal/solution interface and it has two parts - a compact fixed and a diffuse mobile layers. The mixed potential theory is based on the principle of charge conservation, i.e, the sum of anodic oxidation currents must equal the sum of cathodic reduction currents.
To reinforce learnings in this lecture read pages 100-105
(textbook)
To prepare yourself for the next lecture
read pages 107-110 (textbook)