|
|
[Previous Lecture] [Next Lecture] [Course Outline] [General Info] [Study Guide]
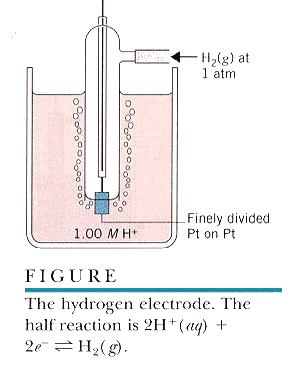
The standard hydrogen electrode is too complex for routine use in laboratory and field applications.
Simpler reference electrodes:
Saturated Calomel Electrode, SCE
calomel, mercury chloride, Hg2Cl2
SCE

SHE
SCE is the most commonly used reference electrode in corrosion studies.Some common standard reference electrodes: refer to table in the textbook.
Dry batteries and Daniell cell
Symbolism of a corrosion cell: Zn | Zn2+ || Cu2+ | Cu
Zn electrode on the left, immersed in Zn2+ ions; Cu electrode on the right, immersed in Cu2+ ions. Ionic species are separated by || . Zinc is the anode, copper is the cathode.
The two half-cell reactions are:
The overall reaction Zn + Cu2+ = Zn2+ + Cu
To find the theoretical cell potential, using Nernst equation for each of the half-cell reactions:
E(Zn/Zn2+)=Eo(Zn/Zn2+) - (0.059/2)lg[Zn2+], and
E(Cu2+/Cu)=Eo(Cu2+/Cu) - (0.059/2)lg{1/[Cu2+]}. So the cell potential
E(cell)=Eo(cell) - (0.059/2)lg{[Zn2+]/[Cu2+]}
Convention:
Eo(cell) = Eo(cathode) - Eo(anode) or
Eo(cell) = Eo(right) - Eo(left)
Points to note:
Current flow reduces potential
A system will always react to oppose a change imposed upon it. Le chatelier's principle
The cell potential can be thought of as an ability to supply current. As soon as current is drawn, the ability is reduced.
Thermodynamic can only indicate the tendency of a system to corrode, an equilibrium situation is required and this causes no net flow of current.
Thermodynamics tells us about the tendency of a system to corrode NOT the rate of corrosion.
The rate of a chemical or electrochemical reaction is dependent on the rate constant. The rate constant is related to the activation energy and the temperature of the system.
For a metal M, in equilibrium with its own ions M = M+ + e, the rate of forward reaction (oxidation M=M++e) is exactly balanced by the rate of the reverse reaction (reduction M++e=M), i.e.
ia=ic=io
where i is the rate of reaction expressed in terms of current density and subscripts, a and c, represent the anodic (oxidation) and cathodic (reduction) processes. io is called the exchange current. It is to be noted that exchange current is always associated with an equilibrium condition, that is, the rate of forward (anodic) reaction equals that of the reverse (cathodic) reaction. The net current is zero.
The corrosion current density of a metal is related to the amount of mass reacted by Faraday's Law of electrolysis: i=zFJ, where J=(dM)/(dt), the flux of substance.
In this lecture, we discussed some commonly used reference electrodes. The SCE electrode is most widely used in corrosion measurements. The cell potential can be obtained by subtracting the reduction potential of the anode from the reduction potential of the cathode. A positive cell potential indicates that reaction can take place naturally or spontaneously. The exchange current density is related to the equilibrium condition whereby no net current flows. Faraday's law of electrolysis relates the amount of mass reacted to the corrosion current density.
To reinforce learnings in this lecture read pages 85-95
(textbook)
To prepare yourself for the next lecture
read pages 95-100, 111-120 (textbook)