|
|
[Previous Lecture] [Next Lecture] [Course Outline] [General Info] [Study Guide]
The law: energy can neither be created nor destroyed.
The Rule: all spontaneous changes occur with a release of free energy from the system to the surroundings at constant temperature and pressure.
Points to note:
In nature:
The driving force for corrosion reaction: chemical energy - energy stored in chemical bonds of substances -internal energy.
Free energy: the portion of internal energy available for powering engines or causes corrosion reaction.
Transition state theory concerns
A+ B= AB= C + D
The transition sate must be of higher free energy than the sum of the free energies of the separate species.
The rate of corrosion reaction
![]()
The quantity in square brackets is a measure of the amount of substance. This will be explained in the next section. The rate constant can be shown to be related to the size of the free energy barrier:
![]()
where C and R are constants, and T is the absolute temperature. Inspection of the equation shows
that as T increases, so also does the rate constant (and hence the rate) but when the size of the barrier (![]() ) is increased, the rate constant decreases. Eqn.
(2.3) is a modified form of an important equation called the Arrhenius equation, named after the scientist who
first described this successful theory.
) is increased, the rate constant decreases. Eqn.
(2.3) is a modified form of an important equation called the Arrhenius equation, named after the scientist who
first described this successful theory.
Now consider the reverse process. Overall, it cannot be spontaneous, because there is an increase
in free energy upon conversion of C and D into A and B. Furthermore, there is a bigger free energy barrier for
the new reactants, C and D, to cross. The Transition State Theory says that the reverse process is possible, but
occurs at a much reduced rate, represented by an equation similar to eqn. (2.3) in which the activation free energy
has been increased from ![]() to (
to ( ![]() +
+ ![]() ),
as in Fig. 2.1. The reverse process is possible
only on the molecular scale where the energies of an individual C and D pair may be such as to allow the formation
of the transition state. (Remember that our rule applies to an overall free energy change for a bulk system.) The
reverse process occurs at a rate far less than the rate of the forward process, so the net reaction observed on
the large scale will always appear to be a steady conversion of A + B into C + D. For the reverse process to occur
in the bulk system, energy must be supplied to the system (e.g. in electrolysis).
),
as in Fig. 2.1. The reverse process is possible
only on the molecular scale where the energies of an individual C and D pair may be such as to allow the formation
of the transition state. (Remember that our rule applies to an overall free energy change for a bulk system.) The
reverse process occurs at a rate far less than the rate of the forward process, so the net reaction observed on
the large scale will always appear to be a steady conversion of A + B into C + D. For the reverse process to occur
in the bulk system, energy must be supplied to the system (e.g. in electrolysis).
The use of energy profiles is of considerable assistance in the understanding of corrosion processes and will be
frequently used throughout our discussions of corrosion theory. We shall now progress to a description of substances.
Fact: 103 elements have been discovered;
Five of the 103 elements: oxygen, silicon, aluminum, iron and calcium make up 91% of
the matter in the earth's crust. The amounts of oxygen and silicon together make 74.3% of the mass.
Of the 103 elements, the great majority are metals.
amount of substance:
One mole of a substance is the molecular weight of that substance expressed in grams.
One mole of material A and one mole of material Bhave equal numbers of molecules but different masses.
Three ways of atoms may combine:
By losing an electron, the atom is ionized.
Cation: the ion with a net positive charge.
By gaining an electron, the atom is ionized.
Anion: the ion with a net negative charge.
Oxidation reaction is related to
Reduction reaction is related to
M = Mn+ + ne- fundamental definition of corrosion:
corrosion is the degradation of a material by an electrochemical reaction with its environment.
Dissociation of water into hydrogen ions and hydroxyl ions:
H2O = H+ + OH- (2.8)
Law of mass action:
For equilibrium process
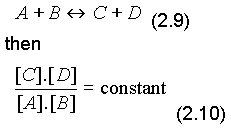
applying law to equilibrium reaction (2.8):
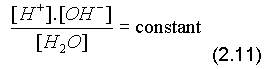
[H+].[OH-] = constant
Under standard conditions: 25oC, 1 atmosphere, [H+].[OH-] = 10-14
are always more than willing to exploit the might of metals,
corrosion engineer are required to patch up the blight of corrosion.
A profitable career ???
Properties of metals:
Metals are extremely important in modern engineering yet many are susceptible to corrosion.
What makes them so useful yet so vulnerable ?
Solidification process determines:
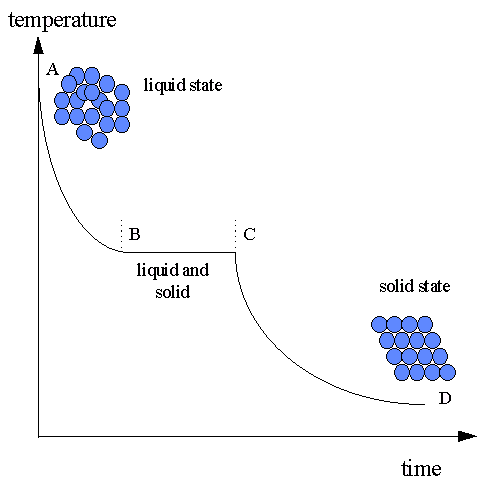
A typical cooling curve for the solidification of a metal. Alloys show similar behaviour with discontinuities at B abd C (known as 'arrests') but which are not at the same temperature.
Effect of cooling rate on
Rapid cooling causes many nucleation sites; slow cooling yields a few crystals.
Dendrite: a branched crystal
Dendritic growth: process of solidification to form dendrite.
Grains: individual crystals
Grain boundaries: zones between any two grains
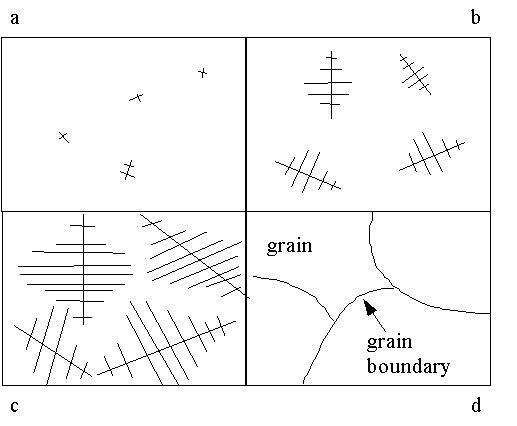
Fig.2.4 Dendritic growth and solidification: (a) nucleation of crystals in the melt; (b) growth of crystals into dendrites; (c) complete solidification; and (d) final grain structure.
Imperfections
in the lattice structures of metals known as defects, have a considerable effect on the corrosion resistance properties.
Types of defects occur within the grains:
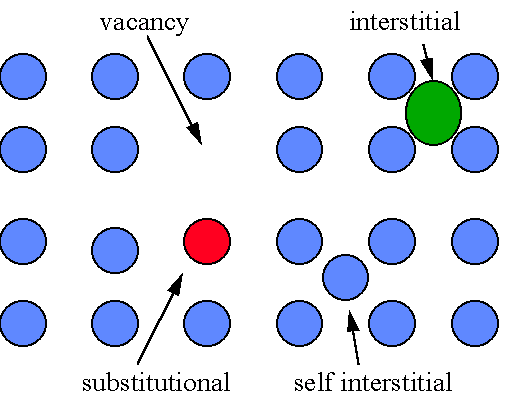
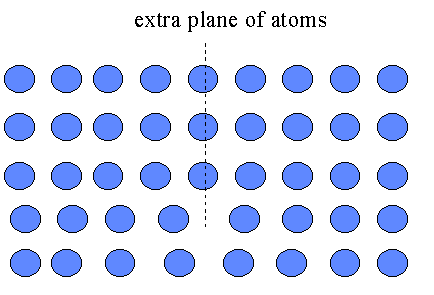
2. Line defects: -planes of atoms not perfectly fitted into the lattice(dislocations)
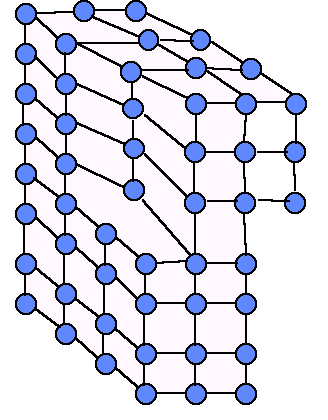
edge dislocations screw dislocation
3. Volume defects
Volume defects play an important role in corrosion mechanisms.
Electricity: passage of the charged particles between two defined points.
Current: the flow of electrons
Resistance: opposition to the flow of electrons
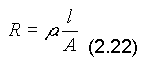
A: cross sectional area
l: length of conductor
p: resistivity/specific resistance
Conductance: the reverse of resistance
Types of electric conductors:
Directions:
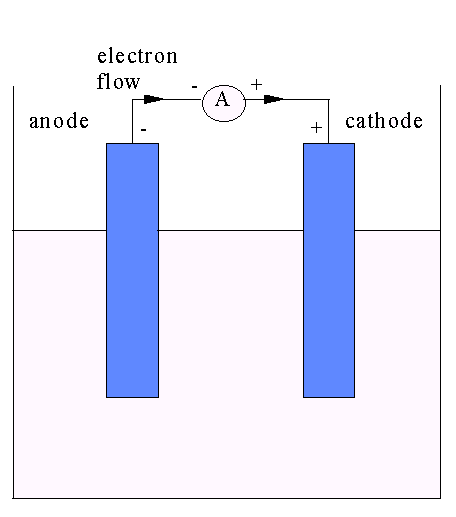
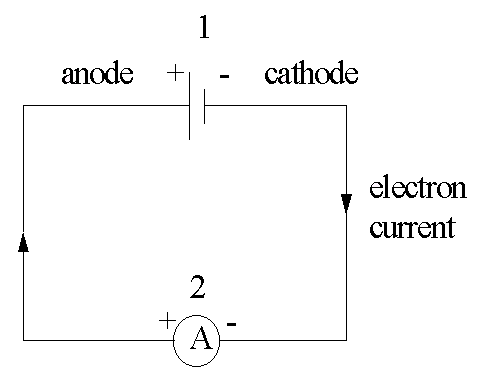
Electromotive force E. M. F.series: driving force (electrochemical potential) from a cell.
Volts = Amperes x Ohms
V=IR
In this lecture,we discussed some of the enabling theories for aqueous corrosion. The basic concepts covered are: chemical, metallurgical and electrical in nature. As the interaction between a material with its environment is affected by both the chemical parameters associated with the environment (pH, temperature, flow rate, dissolved gases etc.) and the metallurgical factors associated with the material (procesing conditions, heat history, defects and inclusions etc), it is not difficult to realize that the environment is just as important as the material in the context of corrosion.
To reinforce learnings in this lecture read pages 23-45
(textbook)
To prepare yourself for the next lecture
read pages 69-81(textbook)